Ammonia/Water - Absorption Refrigeration System
Some liquids like water have a great tendency for absorbing large amount of certain vapors (NH3) and reduce the total volume quite. The absorption chiller refrigeration system differs basically from vapor compression system only in the method of compressing the refrigerant.
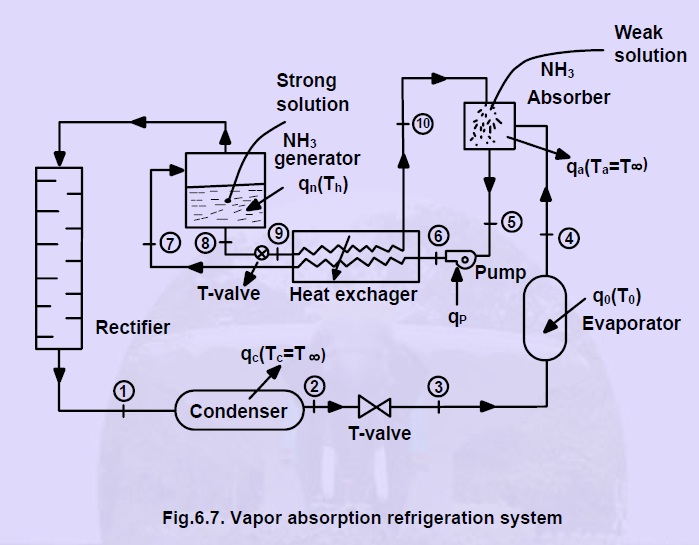
In the absorption refrigerating system, the compressor is replaced with an absorber, generator and pump. Figure shows the schematic diagram of a vapor absorption refrigeration system.
Ammonia vapor is extracted from the NH3 strong solution at high pressure in the generator by an external heating source. In the rectifier, the water vapor which carried with ammonia is removed and only the dried ammonia gas enters into the condenser, where it’s condensed.
The pressure and temperature of cooled NH3 solution is then reduced by a throttle valve below the temperature of the evaporator. The NH3 refrigerant at low temperature enters the evaporator and absorbs the required heat from it, then leaves it as saturated vapor.
The low pressure NH3 vapor is then passed to the absorber, where it’s absorbed by the NH3 weak solution which is sprayed also in the absorber as shown in Fig. After absorbing NH3 vapor by the weak NH3 solution (aqua–ammonia), the weak NH3 solution becomes strong solution and then it is pumped to the generator passing through the heat exchanger.
In the pump, the pressure of the strong solution increases to generator pressure. In the heat exchanger, heat form the high temperature weak NH3 solution is absorbed by the strong NH3 solution coming from the absorber.
As NH3 vapor comes out of the generator, the solution in it becomes weak. The weak high temperature NH3 solution from the generator is then passed through the throttle valve to the heat exchanger. The pressure of the liquid is reduced by the throttle valve to the absorber pressure.
Why Do We Use Ammonia/Water ?
Because most commercial and industrial refrigeration applications occur at temperatures below 32 F and many are 0 F. As a result, a fluid which is not subject to freezing at these temperatures is required. So the lithium bromide/water cycle is no longer able to achieve this conditions, because water is used for the refrigerant.
Also the required heat input temperatures must be at least 230oF. It should also be remembered that the required evaporation temperature is 10 to 15oF below the process temperature.
Use of ammonia/water equipment in conjunction with geothermal resources for commercial refrigeration applications is influenced by some of the same considerations as space cooling applications. Figure 13.5 illustrates the most important of these. As refrigeration temperature is reduced, the required hot water input temperature is increased.