Adsorption
Adsorption is a process that occurs when a gas or liquid solute accumulates on the surface of a solid or a liquid (adsorbent), forming a film of molecules or atoms (the adsorbate). The term sorption encompasses both processes, while desorption is the reverse process of adsorption.
Desorption
Desorption is a phenomenon whereby a substance is released from or through a surface. The process is the opposite of sorption (that is, adsorption and absorption). As the temperature rises, so does the likelihood of desorption occurring.
Principles of adsorption refrigeration
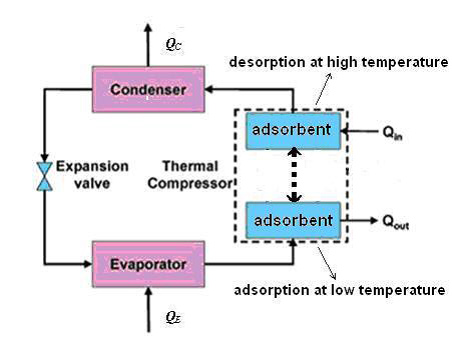
Like the mechanical vapor compression refrigeration cycle and the absorption refrigeration cycle, the adsorption refrigeration cycle can accomplish the removal of heat through the evaporation of a refrigerant at a low pressure and the rejection of heat through the condensation of the refrigerant at a higher pressure.
The pressure difference in the adsorption refrigeration system is created by adsorption and desorption of refrigerant vapor by adsorbent at low temperature and at high temperature respectively.
In comparison with mechanical vapor compression systems, adsorption systems have the benefits of energy saving if powered by waste heat or solar energy, simpler control, no vibration and lower operation costs.
In comparison with liquid absorption systems, adsorption ones present the advantage of being able to be powered by a large range of heat source temperatures, starting at 50℃ and going up to 500℃.
Moreover, the latter kind of system does not need a liquid pump or rectifier for the refrigerant, does not present corrosion problems due to the working pairs normally used, and it is also less sensitive to shocks and to the installation position.