Another class of thermometers that utilise the principle of expansion of substances with temperature is called filled thermal systems. Depending on the phase of the substance, which fills these devices, these systems are sub-categorised into gas-, liquid- and vapour-filled systems.
Gas-filled systems are based on a basic law of gases. If a gas is kept in a metallic bulb (or a container) at a constant volume, then if the temperature varies, so does the pressure according to the relationship
![]()
where:
P1and T1 - absolute pressure (Pa) and temperature (K) at state 1;
P2and T2 - absolute pressure (Pa) and temperature (K) at state 2;
β - thermal coefficient of pressure, equal to the volumetric thermal expansion coefficient,
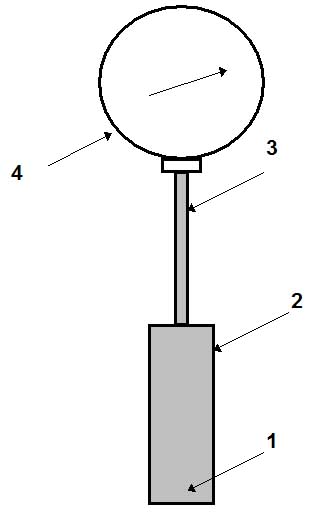
Figure 1: Gas- or liquid-filled thermometer.
Figure 1 schematically shows the design of a gas-filled thermometer. Gas (nitrogen or helium) 1 fills the thermal bulb 2, capillary tube 3 and Bourdon tube of a manometer 4. The thermal bulb (usually made of a stainless steel) is immersed in the measuring media. Variation of its temperature causes change in pressure of the gas in the system. The manometer measures this variation of pressure. The scale of the manometer is graduated in °C, but not in Pa. The length of the capillary tube (usually made of a stainless steel) varies from 0.6 to 60 m. The accuracy of measurements for these thermometers is greatly influenced by variation of ambient temperature (since it can change the pressure of a gas in the system). Two methods are used to reduce this effect:
• a thermal bimetallic temperature compensator is used in the manometer;
• an internal volume of the thermal bulb should be greater than that of the capillary tube, the ratio Vb/Vc (where Vb and Vc are volumes of the thermal bulb and of the capillary, respectively) may vary from 40 to 60; this can be achieved by reducing the internal diameter of the capillary tube or increasing the internal volume of the thermal bulb. The longer the capillary tube, the bigger the thermal bulb should be.
Therefore, gas-filled thermometers are not widely used in practice.
Depending on the measured temperature range, the system may be filled with a gas under pressure higher than atmospheric. That is why variations in atmospheric pressure have no effect on the indications of gas-filled thermometers.
Gas-filled thermometers have several advantages:
• they have the widest temperature range of all filled systems;
• as follows from the equation (1) these thermometers have uniform scales;
• they have the longest capillary length compared with other filled systems.
These thermometers are usually used for temperature measurement in the range from
-200 to 600 °C.
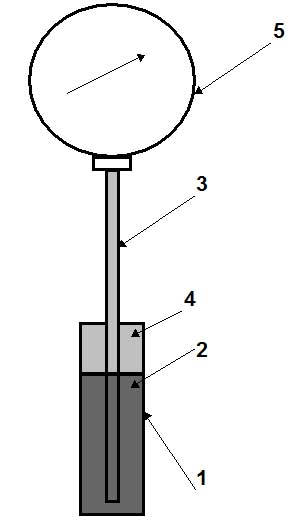
Figure 2: Vapour-pressure system.
Liquid-filled systems have similar design with gas-filled thermometers (see Fig. 1). Organosilicone liquids, propanol and mercury are used as thermometric liquids, which fill the entire system. Since the total volume of the thermal system is constant, then variation of temperature of the media, where the thermal bulb is immersed, causes variation in the pressure of the thermometric liquid. This variation in pressure is proportional to the variation of temperature. Therefore, scales of liquid-filled thermometers are uniform.
Several factors influence the accuracy during temperature measurements, namely:
• variation in ambient temperature;
• variation in pressure head;
• variation in atmospheric pressure.
In order to compensate the influence of variation of an ambient temperature it is necessary to increase the ratio internal volume of the thermal bulb/internal volume of the capillary tube, and employ thermal bimetallic compensators (see gas-filled thermometers). The error due to variation of an ambient temperature is bigger in the case of liquid-filled systems, compared to gas-filled systems. Therefore, the capillary length for liquid-filled systems can not exceed 10 m.
When the thermal bulb is placed below or above the manometer, results of such temperature measurements will not be correct. This is because of different pressure head of the liquid column compared with the case when this thermometer was calibrated (the manometer and the thermal bulb were placed on the same level). In this case the error can be eliminated by zero correction of manometer. The ultimate elevation distance between the thermal bulb and the manometer are given in the calibration certificate supplied with the liquid-filled thermometer.
To reduce influence of variation of atmospheric pressure, the system is filled with liquid under pressure from 0.5 to 2.0 MPa.
Here are the advantages of liquid-filled thermometers:
• small time lag;
• small dimensions of thermal bulb.
These thermometers are used for temperature measurement in the range from -150 to 300 °C.
Vapour/pressure systems (see Fig. 2) are filled by 2/3 of the volume of the thermal bulb 1 by liquid 2 which has a low boiling temperature, for example, freon (refrigerant), propylene, acetone, ethylbenzene, methyl chloride, etc. Another (upper) part of the thermal bulb and the capillary tube 3 is occupied by saturated vapour 4 of this liquid. Vapour pressure depends only on the temperature of saturated liquid in the thermal bulb, and therefore, does not depend on the variation of the ambient temperature (this is an advantage). Relationship between saturation pressure and temperature for liquids is non-linear (see Fig. 3). Hence, the scales of these thermometers are non-uniform, with more widely spaced increments at high temperatures. The length of the capillary tube usually does not exceed 25 m.
Disadvantages of vapour-pressure thermometers are as follows:
• narrow temperature range, from -50 to 300 °C;
• slow response time (time lag) of about 20 seconds;
• non-uniformity of the temperature scale.
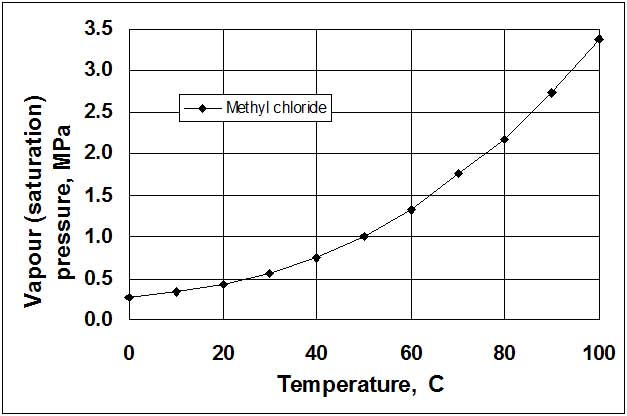
Figure 3:. Saturation (vapour pressure) curve for methyl chloride.