A flame ionization detector (FID) is a scientific instrument that measures analyte in a gas stream. It is frequently used as a detector in gas chromatography. The measurement of ion per unit time make this a mass sensitive instrument.
The operation of the FID is based on the detection of ions formed during combustion of organic compounds in a hydrogen flame. The generation of these ions is proportional to the concentration of organic species in the sample gas stream.
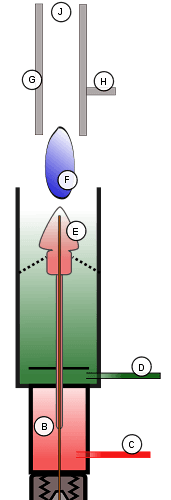
FID Schematic: A) Capillary tube; B) Platinum jet; C) Hydrogen; D) Air; E) Flame; F) Ions; G) Collector; H) Coaxial cable to Analog to Digital converter; J) Gas outlet
FID measurements are usually reported as “as methane”, meaning as the quantity of methane which would produce the same response. Hydrocarbons generally have molar response factors that are equal to the number of carbon atoms in their molecule, while oxygenates and other species that contain heteroatoms tend to have a lower response factor. Carbon monoxide and carbon dioxide are not detectable by FID.
FID measurements are often labelled “total hydrocarbons” or “total hydrocarbon content” (THC), although a more accurate name would be “total volatile hydrocarbon content” (TVHC), as hydrocarbons which have condensed out are not detected, even though they are important for e.g. safety when handling compressed oxygen.
Advantages
Flame ionization detectors are used very widely in gas chromatography because of a number of advantages.
- Cost: Flame ionization detectors are relatively inexpensive to acquire and operate.
- Low maintenance requirements: Apart from cleaning or replacing the FID jet, these detectors require little maintenance.
- Rugged construction: FIDs are relatively resistant to misuse.
- Linearity and detection ranges: FIDs can measure organic substance concentration at very low(10−13 g/s) and very high levels, having a linear response range of 107 g/s.
Disadvantages
Flame ionization detectors cannot detect inorganic substances and some highly oxygenated or functionalized species like infrared and laser technology can. In some systems, CO and CO2 can be detected in the FID using a methanizer, which is a bed of Ni catalyst that reduces CO and CO2 to methane, which can be in turn detected by the FID. The methanizer is limited by its inability to reduce compounds other than CO and CO2 and its tendency to be poisoned by a number of chemicals commonly found in GC effluents.
Another important disadvantage is that the FID flame oxidizes all oxidizable compounds that pass through it; all hydrocarbons and oxygenates are oxidized to carbon dioxide and water and other heteroatoms are oxidized according to thermodynamics. For this reason, FIDs tend to be the last in a detector train and also cannot be used for preparatory work.