Optical Emission Spectrometry (OES) is a fast, accurate and reliable method for quantitative analysis of metals to about 1–3% relative precision.Optical emission spectroscopy methods are among the most useful and flexible means of performing elemental analysis of Steel ,Copper , Aluminium,Magnesium, Lead etc and its alloys. Accurate analysis of metals and alloys requires that :
• The melt or bulk sample is homogeneous;
• Samples are representative of the melt or bulk sample and
• The metallurgical structure of the sample is similar to that of the standards used. The sample requirements are met by chill-cast disk samples collected as described in ASTM E 716-94 (or equivalents AS2612-83 or JISH 1305-1976). For the point-to-plane method of spectrochemical analysis , samples are machined on a lathe to give a smooth, clean surface just prior to analysis.
The sample is mounted in the spectrometer and a spark is generated between the sample and a tungsten electrode. The material is volatilized from the surface of the sample resulting in the emission of light. This light is diffracted into its component wavelengths inside the spectrometer. Fixed detectors simultaneously measure light intensity at wavelengths characteristic of each element.
OES requires calibration of the spectrometer with certified chill-cast standards, which must have the same matrix as the alloys to be tested. Therefore quantitative OES can only be performed on chillcast samples collected from the melt according to standard methods. Other techniques, such as Induction Coupled Plasma (ICP), can be used for quantitative analysis of alloys in other forms, for example billet slices, ingots, extrusions and forgings.
OES PRINCIPLE
The OES system consists of four main parts :
• Sample stand.
• Spark generator.
• Optics assembly.
• Data Acquisition Electronics assembly.
The clean sample is mounted in the stand and a spark is generated between the sample and a tungsten electrode. An example of this setup is illustrated in Figure 1. High purity argon is used as a discharge atmosphere to prevent any interaction between the atmosphere and the sample surface.
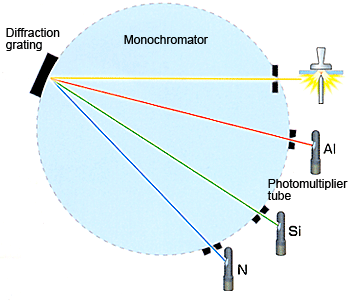
The material is violated from the surface of the sample resulting in the emission of light. When atoms are excited by an external energy source, some electrons move into higher energy levels. As these electrons drop back to their original levels, the atoms emit light (photons) of characteristic wavelength. The spectrometer collects the light emitted from the sample and splits it into its component wavelengths using a diffraction grating.
Fixed detectors (photo multiplier tubes in above figure) simultaneously measure light intensity at wavelengths characteristic of each element. This is illustrated in Figure above. The intensity of the signals depends on the number of photons produced per unit time. The spectrometer is programmed for fixed sample types, elements and concentration ranges and only give results within the limits of calibration .