Inside a maze of silver towers and pipes is a fascinating factory that changes hydrocarbon molecules to make gasoline.
A refinery is a factory. Just as a paper mill turns lumber into legal pads or a glass works turns silica into stemware, a refinery takes a raw material–crude oil-- and transforms it into gasoline and hundreds of other useful products. A typical large refinery costs billions of dollars to build and millions more to maintain and upgrade. It runs around the clock 365 days a year, employs between 1,000 and 2,000 people and occupies as much land as several hundred football fields. It’s so big and sprawling, in fact, that workers ride bicycles from one station to another.
Gasoline’s lowly status rose quickly after 1892, when Charles Duryea built the first U.S. gas-powered automobile. From then on, the light stuff from crude oil became the right stuff.
Today, some refineries can turn more than half of every 42-gallon barrel of crude oil into gasoline. That’s a remarkable technological improvement from 70 years ago, when only 11 gallons of gasoline could be produced. How does this transformation take place? Essentially, refining breaks crude oil down into its various components, which then are selectively reconfigured into new products. This process takes place inside a maze of hardware that one observer has likened to “a metal spaghetti factory.” Employees regulate refinery operations from within highly automated control rooms. Because so much activity happens out of sight, refineries are surprisingly quiet places. The only sound most visitors hear is the constant, low hum of heavy equipment.
The complexity of this equipment varies from one refinery to the next. In general, the more sophisticated a refinery, the better its ability to upgrade crude oil into high-value products. Whether simple or complex, however, all refineries perform three basic steps: separation, conversion and treatment.
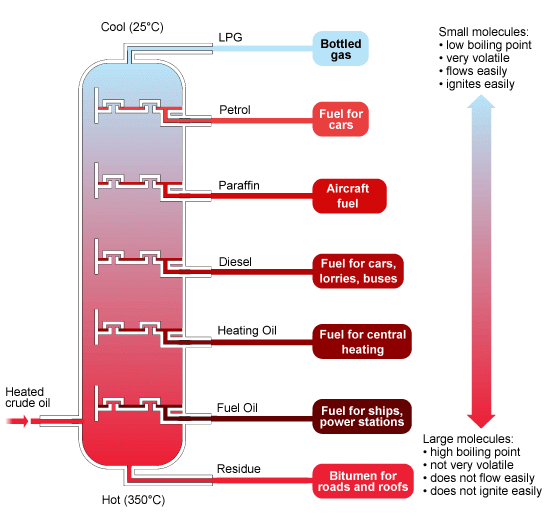
Separation: heavy on the bottom, light on the top
Modern separation–which is not terribly different from the “cooking” methods used at the Pico Canyon stills–involves piping oil through hot furnaces. The resulting liquids and vapors are discharged into distillation towers, the tall, narrow columns that give refineries their distinctive skylines. Inside the towers, the liquids and vapors separate into components or fractions according to weight and boiling point. The lightest fractions, including gasoline and liquid petroleum gas (LPG), vaporize and rise to the top of the tower, where they condense back to liquids. Medium weight liquids, including kerosene and diesel oil distillates, stay in the middle. Heavier liquids, called gas oils, separate lower down, while the heaviest fractions with the highest boiling points settle at the bottom. These tarlike fractions, called residuum, are literally the “bottom of the barrel.”
The fractions now are ready for piping to the next station or plant within the refinery. Some components require relatively little additional processing to become asphalt base or jet fuel. However, most molecules that are destined to become high-value products require much more processing.
Conversion: cracking and rearranging molecules to add value
This is where refining’s fanciest footwork takes place–where fractions from the distillation towers are transformed into streams (intermediate components) that eventually become finished products. This also is where a refinery makes money, because only through conversion can most low-value fractions become gasoline.
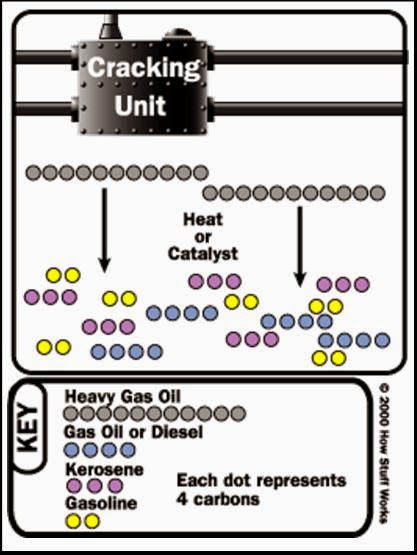
The most widely used conversion method is called cracking because it uses heat and pressure to “crack” heavy hydrocarbon molecules into lighter ones. A cracking unit consists of one or more tall, thick-walled, bullet-shaped reactors and a network of furnaces, heat ex-changers and other vessels.
Fluid catalytic cracking, or “cat cracking,” is the basic gasoline-making process. Using intense heat (about 1,000 degrees Fahrenheit), low pressure and a powdered catalyst (a substance that accelerates chemical reactions), the cat cracker can convert most relatively heavy fractions into smaller gasoline molecules.
Hydrocracking applies the same principles but uses a different catalyst, slightly lower temperatures, much greater pressure and hydrogen to obtain chemical reactions. Although not all refineries employ hydrocracking, some of industry leader in using this technology to cost-effectively convert medium- to heavyweight gas oils into high-value streams. The company’s patented hydrocracking process, which takes place in the Isocracker unit, produces mostly gasoline and jet fuel.Some refineries also have cokers, which use heat and moderate pressure to turn residuum into lighter products and a hard, coal like substance that is used as an industrial fuel. Cokers are among the more peculiar-looking refinery structures. They resemble a series of giant drums with metal derricks on top.
Cracking and coking are not the only forms of conversion. Other refinery processes, instead of splitting molecules, rearrange them to add value. Alkylation, for example, makes gasoline components by combining some of the gaseous byproducts of cracking. The process, which essentially is cracking in reverse, takes place in a series of large, horizontal vessels and tall, skinny towers that loom above other refinery structures. Reforming uses heat, moderate pressure and catalysts to turn naphtha, a light, relatively low-value fraction, into high-octane gasoline components. Chevron’s patented reforming process is called Rheniforming for the rheniumplatinum catalyst used.